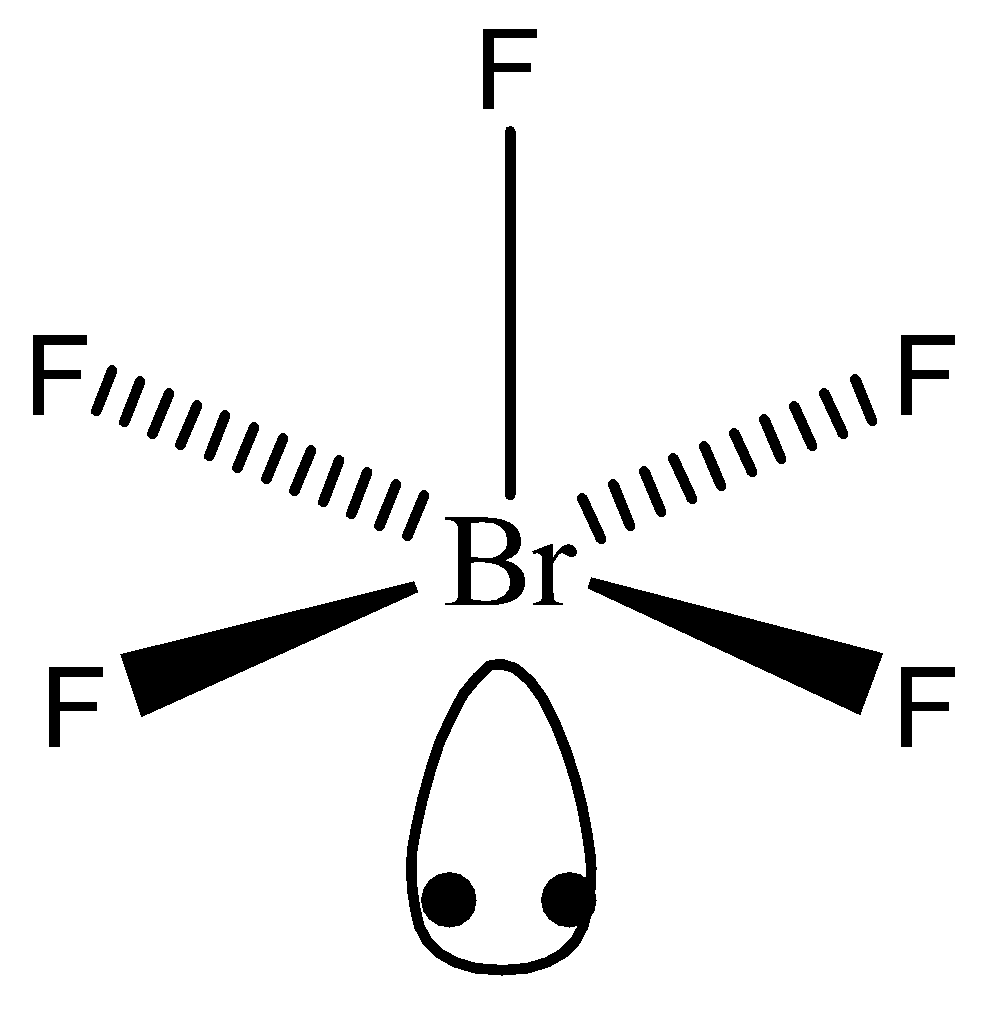
Bromine Pentafluoride Electron Geometry . Brf 5 finds use in oxygen. An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a. 1 shows the various molecular geometries for the five vespr electronic geometries with 2 to 6 electron domains. It is a strong fluorinating agent. Brf5 is a key component in uranium enrichment. Read this article on brf5. When there are lone pairs, you need to look at the structure and recognize the names and bond angles. Have you heard of bromine pentafluoride? When there are no lone pairs the molecular geometry is the electron (vespr) geometry. The brf5 lewis structure is a way to represent the arrangement of atoms and electrons in the molecule. Brf5 is an interhalogen compound as it consists of one bromine and five fluorine atoms. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine.
from www.vedantu.com
Brf 5 finds use in oxygen. When there are lone pairs, you need to look at the structure and recognize the names and bond angles. Brf5 is an interhalogen compound as it consists of one bromine and five fluorine atoms. 1 shows the various molecular geometries for the five vespr electronic geometries with 2 to 6 electron domains. It is a strong fluorinating agent. Brf5 is a key component in uranium enrichment. Read this article on brf5. An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a. The brf5 lewis structure is a way to represent the arrangement of atoms and electrons in the molecule. When there are no lone pairs the molecular geometry is the electron (vespr) geometry.
What is the geometry of the molecule of bromine pentafluoride?A.Square
Bromine Pentafluoride Electron Geometry 1 shows the various molecular geometries for the five vespr electronic geometries with 2 to 6 electron domains. The brf5 lewis structure is a way to represent the arrangement of atoms and electrons in the molecule. Brf 5 finds use in oxygen. Have you heard of bromine pentafluoride? 1 shows the various molecular geometries for the five vespr electronic geometries with 2 to 6 electron domains. When there are lone pairs, you need to look at the structure and recognize the names and bond angles. An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a. It is a strong fluorinating agent. Brf5 is a key component in uranium enrichment. Brf5 is an interhalogen compound as it consists of one bromine and five fluorine atoms. When there are no lone pairs the molecular geometry is the electron (vespr) geometry. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. Read this article on brf5.
From www.bigstockphoto.com
Bromine Pentafluorid Image & Photo (Free Trial) Bigstock Bromine Pentafluoride Electron Geometry When there are lone pairs, you need to look at the structure and recognize the names and bond angles. Read this article on brf5. When there are no lone pairs the molecular geometry is the electron (vespr) geometry. Brf5 is a key component in uranium enrichment. An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a. The. Bromine Pentafluoride Electron Geometry.
From www.ch.imperial.ac.uk
VSEPR Theory A closer look at bromine trifluoride, BrF3. Henry Rzepa Bromine Pentafluoride Electron Geometry Have you heard of bromine pentafluoride? The brf5 lewis structure is a way to represent the arrangement of atoms and electrons in the molecule. 1 shows the various molecular geometries for the five vespr electronic geometries with 2 to 6 electron domains. Read this article on brf5. Brf 5 finds use in oxygen. Brf5 is an interhalogen compound as it. Bromine Pentafluoride Electron Geometry.
From pnghero.com
Bromine Pentafluoride Lewis Structure Molecular Geometry Molecule PNG Bromine Pentafluoride Electron Geometry Brf 5 finds use in oxygen. Brf5 is a key component in uranium enrichment. The brf5 lewis structure is a way to represent the arrangement of atoms and electrons in the molecule. An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a. When there are lone pairs, you need to look at the structure and recognize the. Bromine Pentafluoride Electron Geometry.
From www.youtube.com
BrF5 Molecular Geometry & Bond Angles (Bromine Pentafluoride) YouTube Bromine Pentafluoride Electron Geometry The brf5 lewis structure is a way to represent the arrangement of atoms and electrons in the molecule. Have you heard of bromine pentafluoride? Brf5 is an interhalogen compound as it consists of one bromine and five fluorine atoms. Brf5 is a key component in uranium enrichment. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of. Bromine Pentafluoride Electron Geometry.
From imgbin.com
Bromine Pentafluoride Bromine Trifluoride Lewis Structure Bromate PNG Bromine Pentafluoride Electron Geometry Have you heard of bromine pentafluoride? Brf5 is an interhalogen compound as it consists of one bromine and five fluorine atoms. Brf 5 finds use in oxygen. When there are no lone pairs the molecular geometry is the electron (vespr) geometry. Brf5 is a key component in uranium enrichment. 1 shows the various molecular geometries for the five vespr electronic. Bromine Pentafluoride Electron Geometry.
From www.wikiwand.com
Bromine pentafluoride Wikiwand Bromine Pentafluoride Electron Geometry Read this article on brf5. Have you heard of bromine pentafluoride? Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. Brf5 is an interhalogen compound as it consists of one bromine and five fluorine atoms. An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a. Brf 5 finds use in oxygen. Brf5. Bromine Pentafluoride Electron Geometry.
From www.chegg.com
Solved Electron Geometry Molecular Geometry Bromine Bromine Pentafluoride Electron Geometry Brf5 is an interhalogen compound as it consists of one bromine and five fluorine atoms. Have you heard of bromine pentafluoride? The brf5 lewis structure is a way to represent the arrangement of atoms and electrons in the molecule. It is a strong fluorinating agent. When there are lone pairs, you need to look at the structure and recognize the. Bromine Pentafluoride Electron Geometry.
From www.youtube.com
BrCl5 Molecular Geometry, Bond Angles (and Electron Geometry) YouTube Bromine Pentafluoride Electron Geometry The brf5 lewis structure is a way to represent the arrangement of atoms and electrons in the molecule. When there are lone pairs, you need to look at the structure and recognize the names and bond angles. It is a strong fluorinating agent. 1 shows the various molecular geometries for the five vespr electronic geometries with 2 to 6 electron. Bromine Pentafluoride Electron Geometry.
From askfilo.com
What is the geometry of molecule of bromine pentafluoride? [MH CET 2014].. Bromine Pentafluoride Electron Geometry Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. Brf 5 finds use in oxygen. Brf5 is a key component in uranium enrichment. It is a strong fluorinating agent. An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a. 1 shows the various molecular geometries for the five vespr electronic geometries with. Bromine Pentafluoride Electron Geometry.
From chemistry-europe.onlinelibrary.wiley.com
Bromine Pentafluoride BrF5, the Formation of [BrF6]− Salts, and the Bromine Pentafluoride Electron Geometry When there are lone pairs, you need to look at the structure and recognize the names and bond angles. It is a strong fluorinating agent. Brf5 is a key component in uranium enrichment. Brf5 is an interhalogen compound as it consists of one bromine and five fluorine atoms. When there are no lone pairs the molecular geometry is the electron. Bromine Pentafluoride Electron Geometry.
From ajorpng.blogspot.com
Chlorine Pentafluoride Molecular Geometry / De wikipedia, la Bromine Pentafluoride Electron Geometry Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. Brf5 is a key component in uranium enrichment. When there are lone pairs, you need to look at the structure and recognize the names and bond angles. The brf5 lewis structure is a way to represent the arrangement of atoms and electrons in the molecule. Have. Bromine Pentafluoride Electron Geometry.
From www.chegg.com
Solved Draw the Lewis structure of bromine pentafluoride, Bromine Pentafluoride Electron Geometry Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. Brf 5 finds use in oxygen. When there are lone pairs, you need to look at the structure and recognize the names and bond angles. When there are no lone pairs the molecular geometry is the electron (vespr) geometry. It is a strong fluorinating agent. 1. Bromine Pentafluoride Electron Geometry.
From learningfullspacier.z14.web.core.windows.net
How To Use The Vsepr Theory Bromine Pentafluoride Electron Geometry Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. Read this article on brf5. An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a. Brf 5 finds use in oxygen. The brf5 lewis structure is a way to represent the arrangement of atoms and electrons in the molecule. It is a strong. Bromine Pentafluoride Electron Geometry.
From unacademy.com
What is the Hybridization of Bromine Pentafluoride Bromine Pentafluoride Electron Geometry Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a. When there are no lone pairs the molecular geometry is the electron (vespr) geometry. 1 shows the various molecular geometries for the five vespr electronic geometries with 2 to 6 electron domains. The. Bromine Pentafluoride Electron Geometry.
From wellcometreeoflife.org
BrF3 Molecular Geometry (2021) Everything You Need to Know Bromine Pentafluoride Electron Geometry Brf5 is an interhalogen compound as it consists of one bromine and five fluorine atoms. An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a. Brf 5 finds use in oxygen. When there are no lone pairs the molecular geometry is the electron (vespr) geometry. When there are lone pairs, you need to look at the structure. Bromine Pentafluoride Electron Geometry.
From www.slideserve.com
PPT VSEPR and Chemical Polarity PowerPoint Presentation, free Bromine Pentafluoride Electron Geometry Brf 5 finds use in oxygen. Brf5 is an interhalogen compound as it consists of one bromine and five fluorine atoms. When there are no lone pairs the molecular geometry is the electron (vespr) geometry. When there are lone pairs, you need to look at the structure and recognize the names and bond angles. Bromine pentafluoride, br f 5, is. Bromine Pentafluoride Electron Geometry.
From www.vrogue.co
What Is The Hybridization Of Bromine Pentafluoride vrogue.co Bromine Pentafluoride Electron Geometry It is a strong fluorinating agent. Brf 5 finds use in oxygen. When there are no lone pairs the molecular geometry is the electron (vespr) geometry. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. Have you heard of bromine pentafluoride? Brf5 is a key component in uranium enrichment. An explanation of the molecular geometry. Bromine Pentafluoride Electron Geometry.
From br.pinterest.com
VSEPR Theory Molecular and Electron Geometry of Organic Molecules Bromine Pentafluoride Electron Geometry Brf5 is a key component in uranium enrichment. Brf 5 finds use in oxygen. It is a strong fluorinating agent. 1 shows the various molecular geometries for the five vespr electronic geometries with 2 to 6 electron domains. When there are lone pairs, you need to look at the structure and recognize the names and bond angles. When there are. Bromine Pentafluoride Electron Geometry.